Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Metabolic Reprogramming in Nerve Cells
*Corresponding author:Susinjan Bhattacharya, School of Agriculture and Allied Sciences, The Neotia University, India.
Received:February 16,2023; Published:March 09, 2023
DOI: 10.34297/AJBSR.2023.18.002444
Abstract
Mammalian physiology has a complex networked system which is called the nervous system. Nerve cells can undergo degeneration due to injury and stress. Regeneration is a difficult process, and axonal regeneration may succeed or may not. Metabolic changes can lead to the development of many diseases including neurodegenerative diseases. Survival of the metabolically impaired state of the nerve cells is referred to as metabolic reprogramming of the nerve cells in the present review. Metabolic rewiring dependent on intrinsic and extrinsic factors is a necessity for neuron regeneration and can act as curative in neurogenesis. These events highlight the importance of understanding molecular mechanisms of nerve cells metabolism. The knowledge can help us to understand the process of reprogramming in nerve cells that will be useful for nerve cell regeneration. Different plant extracts, or food components or physical activity may help in the process of metabolic reprogramming of nerve cells, but there is a need for scientific studies to understand the process that can support neural regeneration process along with the first line of therapy. The present review discusses neuron metabolism, effects in nervous disorders, and suggests possible interventions that can be exercised as therapeutic interventions.
Keywords: Neural metabolism, Metabolic transitions, Glial cells, Astrocytes, Neurodegenerative diseases, Neural regeneration, Dietary components, Nerve growth factors
Introduction
The mammalian system is composed of different types of systems and networks, and one of the communicating systems is the nervous system. The nervous system along with the endocrine system is responsible for maintaining homeostasis in the human body [1]. The fundamental unit of the nervous system is neuron, or also called as ‘nerve cell’. Apart from neurons, the nervous system also bears neuroglial cells. The nerve cell carries electrical impulses, and consists of dendrite, axon and a cell body and communication between nerve cells occurs by structures called ‘synapse’ [2]. The nervous system is not only responsible for the cognitive functions in our body but also the movement, digestion, and other activities [3]. The nervous system in vertebrates is divided into two categories, central nervous system (CNS) and peripheral nervous system (PNS) [4].
Impairment of functioning of the nervous system and nerve cells leads to the generation of neurodegenerative disease. Functional impairment of neurons and behavioral abnormalities are evoked by neural cells [5]. Neural non-coding RNAs play a critical regulatory role in brain functions [6]. Neurodegenerative diseases occupy an important viewpoint in public health because of the complexity involved in prevalence, time, and cost in treatment. The prevalence is due to a reason of multiple factors, and one of these is due to the lifestyle of an individual. Effective treatment for neurodegenerative diseases is difficult, and this gives the importance to finding out new therapeutic interventions. Metabolic factors play prime in the development of many complex diseases, and the same cause lies in the development of neurodegenerative diseases [7,8]. Low nutrient and oxygen conditions leads to an environmental adaptation of the cells, primarily tumour cells by the process called as metabolic rewiring, whereas metabolic plasticity refers to the ability of the cells to adapt their metabolic status to specific needs during development, differentiation, or response to stimuli [9-11].
Again, metabolic reprogramming used in context to tumour cell metabolism refers to ability of tumour cells to alter their metabolism for growth and energy requirements [12]. The mechanism of metabolic plasticity meets gene regulation, which is observed in metabolic rewiring also [13]. Metabolic functions of nerve cells alter neurodegeneration and ageing and other pathological conditions. This can also be an adjustment of the nerve cells to grow and sustain themselves under different pathological situations. Thus, here in this work, the survival of metabolically impaired state of nerve cells is referred to as ‘metabolic reprogramming’ of nerve cells. The situation can be altered or enhanced by different factors that can be intrinsic, or extrinsic like diet, exercise, etc. The concept of metabolic reprogramming emphasizes on the need for development of therapeutic strategies that can be based on drugs, food, lifestyle, exercise, and social interactions in cure for neurodegenerative diseases [14]. The present work within the limited scope is looking at the metabolic aspects in functioning and dysfunctioning of the nerve cells and suggest on the possible interventions that can be exercised as therapeutic interventions.
Neural Metabolism
Understanding metabolism of the neurons can unravel reasons behind nerve cell damages as in stroke, memory loss as well as in Parkinson’s and Alzheimer’s disease [15]. Brain neurons together with astrocytes possess a high energy demand [16].
Microglia is understood to be a reactive contributor in neurological disorders [5]. Growth of dendrite, a highly branched network system in human brain is a complex process and is controlled by a few signaling pathways. In this process, active Nedd4-1 prevents protein degradation by ubiquitylation, and dendrites can grow due to the functions played by Nedd4/Rap2/ TNIK [17].
In situations of injury to the brain, nerve cells cannot be replenished, as they cannot renew themselves. However, in events of stroke, treatment with stem cells shows a promising approach [18]. Furthermore, nerve growth factors, or neutrophins also help in the regeneration process [19].The transcription factor family, E2F playing role in cell cycle also plays important role in neuronal differentiation, and E2F4 is important for terminal differentiation of neuroblasts [20]. Communication between neurons demands high energy, and glucose supply can vary in the nerve cells. Excess concentration of glucose can lead to mitochondrial inactivation in the neurons [21]. The process can lead to abnormal neuronal firing and axonal shearing and consequently death of the neurons. This is seen in Alzhemier’s disease, as well as in Dementia, and ability of a person to understand and respond to any signal comes down [22]. Energy demand in the brain rises with large number of neurons and is essential to maintain functioning of the neurons. Bulk of the energy consumption occurs at synapses, which is balanced by the activity of Na+/K+ ATPase pump with regulation of other ions like Ca2+, Mg2+, HCO3-, Cl- and H+. Astrocytic end feet mediate supply of glucose to the brain cells. Astrocytes can store glucose as glycogen or convert glucose to lactate and supply lactate to neurons by Glucose transporters (GLUT 3) for conversion to pyruvate and entrance to the citric acid cycle. Additionally, glucose can directly enter neurons by GLUT 3 for generation of pyruvate by glycolysis or enter to pentose phosphate pathway (PPP) to generate NADPH and other molecules [23]. Intermediates from PPP act as nucleotide precursors, whereas intermediates from glycolysis can serve as precursors for many biosynthetic metabolic pathways [24]. Lactate shuttle in brain occurs by mono-carboxylate transporters, key molecules for interaction between glia and neurons mediated by carbonic anhydrases (CA), playing role in bicarbonate transport [23,25]. Glucose reaches brain astrocytes, oligodendrocytes via GLUT 3 and microglial cells via GLUT 5 [26]. Glycogen is unevenly distributed in the brain, and concentrations are highest in regions with the highest synaptic density [27,28]. During stress, or any activity requiring more energy, glycogen from astrocytes is immediately converted to lactate, and lactate provides neuro protective effect against hypoglycemia, or ischemia [25]. Synaptic activity also involves uptake of K+ ions by astrocytes released by neurons with energy derivation from the breakdown of glycogen reserves [29-32]. Neurons can increase their glycolytic ability in response to stimulation and the metabolic resupply of energy in neurons can be regulated by feedback signaling by adenosine diphosphate and feedforward signaling by calcium ions. The choice between glycolysis and oxidative phosphorylation has an important effect on health and disease. However, during brain stimulation there is transient uncoupling between glycolysis and oxidative phosphorylation [33]. Astrocytes can flourish under hypoxia and depend on glycolysis, whereas neurons depend on oxidative metabolism and can succumb to ischemia [34]. Axonal myelination and regeneration are supported by monocarboxylate transporters [35]. Apart from glucose and lactate, acetate also serves as energy source to nerve cells. ATP generation in the brain is triggered mainly by glucose metabolism, and glucose supply to the brain is controlled by neurovascular coupling, and cross blood brain barrier by GLUTs. Disturbances in glucose metabolism leads to many neurological diseases [36,37]. Disruption of monocarboxylate transporters can also lead to neurodegeneration. The peripheral nervous system depends on lactate as fuel source [38-42]. Astrocytes, however, use acetate which later enters the citric acid cycle [43-48].
Glucose is needed in resting physiology, whereas lactate and acetate are essential in times of increased neuronal activities, including situations of limited oxygen or glucose supply. However, neuronal stimulation has also been reported to lead to an increased glucose consumption [33,48]. The homeostatic tone of the nervous system is regulated by lactate [49]. Furthermore, peroxisomal β-deficiency leads to metabolic disorder with involvement of the central nervous system (CNS) [5].
Molecular motors apart from playing role in neural disease pathogenesis also play role in brain wiring and neuronal plasticity, as well as regulates CNS and PNS. The molecular motors KIF 3 and KIF17 function for anterograde transport, whereas cytoplasmic dynein 2 plays role in retrograde transport. The myosin family proteins functions in short range transport processes. The KIF3 motor also acts as a tumour suppressor [50,51]. Alterations in the NAD+ level impair the metabolic signaling pathways and lead to different neurodegenerative disorders, ageing and tumourigenesis [52].
Metabolic Interactions
Metabolic interactions between astrocytes and neurons directs brain activity. Disruption of this interaction can lead to the development of generation of neurological diseases. Astrocytes act as sites for L-serine synthesis and mitochondrial reactive oxygen species (ROS) production [24]. Synapse generated glutamate (Glu) and ammoniacal ions (NH4+) is uptaken by the glial cells, and releases glutamine to neurons [53,54]. The functioning of NMDA receptors regulates synaptic plasticity and memory due to D-Serine released from 3-phosphoglycerate (PG) in astrocytes [55,56]. On the other side, L-serine plays a critical role in neurotransmission [57,58]. Simultaneously, glycine synthesized from serine acts as inhibitory neurotransmitter via ionotrophic glycine receptors located primarily in brain stem and spinal cord. The L-serine to glycine conversion coupled to the folate cycle, catalyzed by serine hydroxymethyl transferase leads to the generation of N-5, N-10- methylenetetrahydrofolate (5,10-meTHF) from tetrahydrofolate (THF). L-serine, important for neurotransmission, also provides carbons for the generation of GSH (γ-L-glutamyl-L-cysteinylglycine), an antioxidant [59,60]. Astrocytes with the help of syncytia help in the intra-astrocytic transfer of glucose, water, Ca2+, K+, etc. Immature astrocytes are embedded within astrocytoma which lack neurons [35]. In contrast to neurons, astrocytes can preserve energy stores such as glycogen which later is converted to glucose or lactate [61-63]. Phosphatidylserine, present in neuronal membranes modulates synaptic receptors, proteins, and release of neurotransmitters by exocytosis with Ca2+ -dependent membrane fusion reactions. Impairment of phosphatidylserine functioning results in cognitive impairment and Alzheimer’s disease [64].
Neuron-astrocyte metabolic interaction functions to maintain cellular homeostasis of glutamate, the excitatory neurotransmitter of the brain. Glutamate also plays a role in networking glucose and amino acid metabolism between neurons and astrocytes. Glutamate metabolic shifts lead to the generation of neurodegenerative diseases, like Alzheimer’s disease, Huntington’s disease. Glutamate is recycled between neurons and astrocytes as glutamate and glutamine by glutamate-glutamine cycle [65]. Glutamate also serves as the key metabolite in malate-aspartate shuttle (MAS). The MAS activity transfers cytosolic reducing equivalents of NADH or NAD+ to the mitochondrial matrix, essential for sustained glycolytic activity [66,67]. Dysfunction of glutamate metabolism can lead to neurodegenerative diseases, like Alzheimer’s and Huntington disease. One of the features of Alzheimer’s disease is reduced levels of glutamate levels in the brain, as well as due to disturbed astrocyte glutamine support [68,69].
The blood-brain barrier (BBB), formed by capillary endothelial cells is essential to prevent entry of neurotoxins and pathogens to brain, and functionally interacts with its surrounding of astrocyte perivascular end feet, pericytes and basement membrane [70]. Disturbances in the functioning of BBB leads to loss of synaptic transmission, neuronal connectivity, and functioning [71]. The blood brain barrier is formed by capillary endothelial cells surrounded by basement membrane, pericytes and astrocyte perivascular end feet. Tight junctions between endothelial cells prevent entry of watersoluble molecules, and nutrients can be transported by passive or active-mediated transporters. However, gases and small nonpolar lipids can enter by passive diffusion [70].
Energy Metabolism in Brain
Brain energy metabolism involves networking between energy demand and supply coupled with changes in local blood flow and glucose utilization, also referred to as neurovascular and neurometabolic coupling. Astrocytes play an important role in neurovascular coupling, and astrocyte-neuron interactions regulate cerebral blood flow [72]. Neurons depend on oxidative metabolism to meet their energy needs, whereas astrocytes (glial cells) are highly glycolytic. However, it is the astrocytes that play a critical role in neurotransmitter recycling and anaplerosis, and mobilization of astrocytic glycogen stores is critical for long-term memory formation in rats [73-76]. Interaction between astrocyte and neuron is important for defense against oxidative stress, and reduced form of glutathione peroxidase is essential for the detoxification of reactive oxygen species [77].
Metabolic Transitions
Metabolic reprogramming, or transitions occurring in neurons, like changes in oxidative stress and induction of transcriptional sensors of oxidative stress are important in regulating physiological neuronal function, and brain plasticity and cognitive function. Metabolic pathways are disrupted in neurodegenerative diseases and can be due to oxidative stress [78].
Astrocytes have more metabolic plasticity than neurons. Upon nitric oxide exposure, astrocytes elevate their glucose metabolism through glycolysis and thus limiting fall in ATP levels and apoptosis of astrocytes. In contrast, nitric oxide exposure makes neurons lose their ATP levels and apoptosis. If neurons grow in low ATP energy levels, neurons can be deformed leading to death of the cell [15]. Even though metabolic plasticity of astrocytes is beneficial for their beneficial for their homeostatic and neuroprotective functions in physiological conditions, it can turn out to be harmful in pathological conditions [79-81]. Energetic interactions between astrocytes and neurons can also lead to neuronal excitability [72]. Similarly, neuroenergetics or coupling between neuronal activity and energy metabolism plays a critical role in neuron-astrocyte metabolic interactions [82]. Thus, wherein impairment of astrocyte functions can lead to neurodegenerative diseases, restoration of astrocyte function can provide lights for therapeutic opportunities in neurodegenerative diseases [83].
In Alzheimer’s disease, induction of hypoxia leads to an elevation of HIF-1α target, β-site β-amyloid precursor protein cleavage enzyme 1 (BACE 1) that cleaves amyloid precursor protein (APP) to form Aβ. In neuronal mitochondria, oxidative metabolism is disrupted by accumulation of Aβ. This leads to reduction in TCA cycle entry, acetyl-CoA production, α-ketoglutarate dehydrogenase complex, and elevated reactive oxygen species formation and transglutaminase activity leading to increased α-synuclein aggregation with reduction in oxidative respiration [84-90]. Changes in zinc and copper level are coupled to Alzheimer’s disease pathology, and co-localization of copper is seen in Aβ plaques Simultaneously, iron is also co-localized with Aβ-plaques and neurofibrillary tangles [91-97]. Increased iron levels have been reported to be linked with APOE-4 AD risk allele [98]. Ageing is one of the risk factors for developing Alzheimer’s and Parkinson’ s disease (PD). Genetic and environmental factors can also lead to PD and Huntington’s disease (HD). Parkinson’s disease affects patient motor function with formation of α-synuclein aggregates. Huntington’s disease is characterized by formation of expanded CAG repeats in the Huntingtin (HTT) gene leading to neuronal degeneration and cell death in the brain [78]. Development of Parkinson’s disease can result also due to the mutations in mitochondrial genes and exposure to the neurotoxin, MPP+ that inhibits ETC complex I and oxidative respiration leading to permanent Parkinsonism [99-101]. Retention of memory in brain for long term is due to the lactate presence [102]. However, the essential energy substrates during development are ketone bodies, 3-β-hydroxybutyrate (3HB) and acetoacetate (AcAc) [103-105]. Ketone utilization during brain maturation and development is not only essential for energy metabolism, but also for amino acid and lipid metabolism as well as in postnatal period [106,107]. Ketone oxidation in the adult brain increases under conditions of limited glucose availability, wherein ketone bodies are produced in liver from fatty acid and ketogenic amino acid oxidation. In the brain, astrocytes can only generate ketone bodies and uptake of ketone bodies in brain occurs by mono carboxylate transporters [108]. Ketosis in the brain is regulated by blood concentration which is coupled with reduced glucose utilization [109-111].
Consequences of Metabolic Dysfunction
Spontaneous coordinated behavior and nerve cells involves role of gap junction protein, innexin 2 [112]. Ageing reduces gray matter in the brain by dendritic arborization and reduction in synapse numbers. Additionally, white matter density also reduces in the brain with ageing coupled with an increase in the number of white matter lesions and decline in ATP levels. This also leads to structural alterations in mitochondria as well as downregulated association of mitochondria with endoplasmic reticulum [113-116]. Ageing also leads to a gradual decline in energy utilization by the brain, reduced NAD and NAD+ levels and elevated levels of NADH [117,118]. With reduction in ability of the neurons to generate required amount of ATP, synapses initiate their degeneration and dysfunction [119]. Loss of blood-brain-barrier integrity results in parenchymal accumulation of blood-derived proteins and immune cells leading to the development of inflammation [120]. Thus, hypometabolism leads to cognitive dysfunction in ageing as well as the development of adaptive signaling pathways in brain [121]. The neuronal firing patterns leading to neuroanatomical changes result in coginitive deficits with age. Neurons also with age lose their tree branching pattern and turnover [70]. Endothelial transporter proteins are altered in amylotrophic lateral sclerosis (ALS) patients with degeneration of astrocyte endfeet and abnormal level of blood protein in the cerebrospinal fluid. There is also deposition of IgG and complents in spinal cord and motor cortex [122-126]. The ALS patients also exhibit glucose intolerance, insulin resistance and hyperlipidemia [127-129]. Impairment of mitochondrial functions in mitochondria results from the mutations in the genes encoding α-synuclein, Parkin (Park 2), PTEN induced putative kinase-1 (PINK1), Leucine rich repeat kinase-2 (LRRK2), DJ-1 (Park 7) [130].
Sleep apnea
The healthcare issues in neurological patients can also lead to the development of obstructive sleep apnea [131]. Obstructive sleep apnea (OSA) is a complex disease related to the dysregulation of the molecular clock and other associated biological processes [132]. The hypothesis being put forth in OSA is that the monoaminergic neurons (noradrenergic and serontonergic) inhibit cholinergic neurons leading to suppression of REM sleep and upregulating NREM sleep. This is due to the decrease in firing frequency of monoaminergic neurons in NREM sleep, and increase in firing of cholinergic neurons in REM sleep. The process leads to the development of a recurring cycle [133]. Sleep apnea is associated with many other complications including Down’s syndrome [134]. Understanding molecular biology of sleep apnea can lead to the development of new treatments [135]. There are a few reports that have shown the molecular domains affected in OSA, adipokines, celladhesion molecules and molecules responding to the endoplasmic reticulum stress [136].
Reprogramming
The cellular and molecular mechanisms of cognitive functions need to undergo more research. Though there are several reports on neuronal reprogramming, the mechanisms of cell fate conversions are not being well studied. Reports observe the coexpression of Bcl-2 and anti-oxidative treatments to improve glial to neuron conversion after traumatic brain injury [137]. The neural transcription factor, Neuro D1 could also reprogram glial cells to functional neurons, as well as human cortical cells to functional neurons [138]. Furthermore, the transcription factor ATF3 induced after early peripheral nerve injury developed cellular plasticity in sensory neurons to transform into a regenerative state [139]. Additionally, animal models have shown that Muller glia in zebrafish can be reprogrammed to regenerate retinal neurons [140].
In Drosophila, gut derived cytokines metabolically reprogrammed glial cells [141]. Glia has also been evidenced to undergo metabolic reprogramming in amyotrophic lateral sclerosis (ALS) due to mitochondrial dysfunctions [142]. Synaptic plasticity is a critical mechanism at the neuronal level [143]. Research work evidences the role of astrocytic ApoE to reprogram lipid metabolism and regulate neuronal epigenetic conditions. Brain function with memory activity is modulated in the process. However, comparatively ApoE4 is able to reprogram neurons than ApoE3 [144].
Neural Regeneration
Nerve injuries can result in loss of body parts. Regeneration is a difficult process, and stem cell replacement therapy is one of the possible interventions. Studies also suggest the use of growth factors in regeneration of the nerve cells [145]. Neurological dysfunctions and neurological disease are associated with alterations in neural stem cell (NSC) differentiation [146]. The molecules, p53 and p73, play key role in brain development and regulation of CNS development, and transition of GABA from excitatory to inhibitory coupled with glutamate excitatory inputs plays critical role in neurogenesis [147-151]. The mTOR (mammalian target of rapamycin) signaling plays an important role in differentiation, neurite outgrowth and synaptic formation, whereas HDAC inhibitors act as neuroprotective in neurodegeneration [152-154]. In summary, extrinsic, and intrinsic factors regulate neurogenesis and impairment of neurogenesis contributes to development of neurodegeneration and cognitive impairment [155]. Of the important players in synaptic circuit, gap junction protein connexin-36 plays a critical role [156].
Furthermore, nerve growth factor, a target derived neutrophic factor essential for the survival, growth and functioning of the peripheral and central nervous system also plays role in chemotactic movement of rat peritoneal mast cells by influencing survival and differentiation of the mast cells. The mechanism involves drastic morphological change and distribution of F-actin fibres and use of mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathway [157]. Different types of cells of the central nervous system arise from the ability of neural stem cells to undergo symmetric divisions and proliferation, as well as differentiation by asymmetric divisions. The NSCs generate neural precursor cells that again give rise to functional neurons in embryonic neuron development and adult central nervous system. The process for generation of pluripotentiality in NSCs to create young neurons called as neurogenesis, involves development of neuroblasts which differentiate to new neurons that migrates to the existing neural networks due to regulation by intrinsic and extrinsic signals [158,159]. The extrinsic factors includes transcriptional regulators, Sonic Hedgehog (Shh), Notch, Wnt, Bone morphogenetic proteins (BMP), Oct4, Sox2 and Nanog, whereas the intrinsic factors includes epigenetic regulators [160]. Interaction between microRNAs (miRNA) and nuclear receptor TLX plays role in dedifferention process of NSCs. Crosstalk between let-7b and cyclin D1 through TLX initiates stages of development [161,162]. Additionally, neurogenesis is mediated by growth factors and neutrophic factors. The identified neutrophic factors playing role here are four, namely nerve growth factor (NGF), BDNF, neurotrophin 3(NT-3), and neurotrophin 4/5(NT-4/5), wherein neutrophins binds to tyrosine kinase receptors [163-166].
Neural transcription factors are involved in the development of neural stem cells right from the embryonic stage [167]. For development of brain, subgroup of the basic-helix-loop-helix (bHLH) transcription factor, a neural lineage bHLH transcription factor, Neurod family is essential for development of CNS. The participating members of the Neurod family are four, namely Neurod 1, Neurod 2, Neurod 4 and Neurod 6 [168].
The bHLH transcription factor, NeuroD1, critical to initiate neuronal development program can reprogram other cell types to neurons. NeuroD1 binds directly to the regulatory elements of neuronal genes that can be developmentally silenced by epigenetic mechanisms [169]. The LIM homeodomain transcription factor, ISL1 plays a key role in the development of sympathetic nervous development by controlling cell cycle gene expression. This is essential for glial differentiation repression and neuronal differentiation [170].
Neural morphogenesis is regulated by the cell-adhesion molecules and actin-associated proteins. The movement mechanism of nerve growth cones with actin fibers determines axonal pathfinding in embryogenesis [171].
Long distance signaling in neurons is mediated by calcium signaling and bidirectional transport of proteins, vesicles, and mRNAs along microtubules. This is also helped by the axonal lengths and characteristic feature of the neurons being polarized cells. The process mediates in communication about axon injury to the soma, so as to enable initiation of the repair mechanisms [172]. Glial cell derived neurotrophic factor has been observed not only to prolong survival and induce enteric neurogenesis, but also improve colon structure and function in Hirschsprung disease [173]. Reports also observe the use of exosomes for the treatment of peripheral nerve injury [174].
The fibroblast growth factor (FGF) signaling is responsible for the development of spinal cord [175]. Insulin Growth factor 1 (IGF) signalling plays role in adult neurogenesis, as well as stimulating differentiation of adult hippocampal progenitor cells to oligodendrocytes by inhibiting BMP signaling [176]. Vascular endothelial growth factor (VEGF) functions in the regulation of growth and maturation of neurons during development [177,178]. The three growth factors, FGF, IGF and VEGF functions by associating with tyrosine kinases [146]. Microenvironmental resource competition regulates differentiation of oligodendrocytes [179]. Neurotransmitters, like glutamate playing critical role in neural communication also function in neurogenesis [180,181]. In contrast to the role of neurotransmitter glutamate, GABA acts as the main inhibitory neurotransmitter in the brain. GABA acts in dual role, either as depolarizer, or as hyperpolarizer but decides based on the intracellular chloride content that decides on the transmembrane gradient [182]. Dopamine is another neurotransmitter of catecholamine involved in ontogenesis and embryonic proliferation of the germinal zone in development [183]. Chemical factors and intercellular contacts also play a critical role in remodeling of the dorsal root ganglion (DRG) cells. Primary sensory neurons are enclosed in DRG, and DRG cells can differentiate to different neuronal subpopulations [184]. In summary, the process of differentiation can be modulated by kinetics of protein metabolism in ganglion cells pregangliotic nerve endings [185].
Axonal Regeneration
Axonal degeneration results from damage to the CNS, and CNS inflammation induces axonal degeneration. Axon growth is inhibited by RhoA. However, stimulation of cyclic adenosine monophosphate (cAMP) by prostacyclin leads to axonal regeneration [186]. Regeneration of axons is signaled by several intrinsic and extrinsic factors [186,187]. The first step of axon regeneration is initiated by growth cone formation. Nonregenerative response leads to the formation of retraction bulbs. Long range anterograde transport helps in axon extension, but in situations of injury, transport gets inhibited and that leads to the regeneration failure [187].
Curative Approaches
Cognitive performance in animals along with neurogenesis and synaptic plasticity improves with dietary energy restriction or fasting and exercise [188]. Experiments in Drosophila melanogaster has shown that short-term fasting results in increased long-term memory, whereas protracted fasting prevents aversive and not pleasant memory formation leading to conclude upon that intermittent bioenergetic changes are good for brain works [189,190]. This phenomenon can be related to the increase in brain functions and neuro protection under metabolic shocks conferring resistance to dysfunction and degeneration [191-193]. Brain ageing is accelerated by sluggish lifestyle, reduced physical and social activity, excessive calories intake, etc., whereas good lifestyle habits with dietary energy restriction, macro- and micronutrients in diet, intellectual and social stimulations, reduction of stress uplifts cognitive abilities in an individual [188]. At the cellular level, neuronal resilience is improved by upregulation of the transcription factors, cAMP response element-binding protein (CREB), nuclear factor κB (NF-κB), and nuclear factor erythroidderived 2 (NRF2) and genetic expression of proteins counteracting cellular stress at multiple subcellular sites, and by different mechanisms [194]. Antioxidant enzymes, antiapoptotic protein, chaperones, neutrophic factors, etc. are upregulated by physical activity and intermittent fasting [194,195]. Adaptive cellular stress response upregulates cytoprotective signaling pathways by secreted neutrophins [196]. Amylin the food intake satiation hormone, and controller of blood glucose levels, and with ability to form amyloid plaques is not only restricted the pancreatic islet cells, but also extends to the CNS, wherein it co-localizes with Aβ-plaques in AD patients. Amylin thus lies at the interface between metabolic and neurodegenerative disorders [197-200]. These suggest that intake of drugs and food promotes brain health development.
Dietary Influence
Food components in terms of dietary intake can help in recovery from neurogenic diseases, or can act as predictive for neurogenic diseases. Of the extrinsic factors to regulate neurogenesis, dietary factor is one of the factors to regulate molecular events in energy metabolism and synaptic plasticity, as well as can complement the cation of exercise [201]. Diet modulates sympathetic nervous system (SNS) role, and intake of sucrose upregulated SNS activity in rat study [202]. Multiple sclerosis (MS) proceeds with the loss of oligodendrocytes and destruction of myelin sheath. Intake of some foods like more of fish, polyunsaturated fatty acids (PUFA), exercising calorie restriction can either decrease, or lead to the development of MS. Adolescent obesity or intake of insufficient vitamin D levels enhance possibility for MS development. Consumption of ketogenic diet can lead to modulate vulnerability to MS [203]. Development of MS may collocate itself with initiation of metabolic syndrome [204- 206]. The situation can aggravate loss of healthy gut microbiota and short chain fatty acid producing bacteria, and encouragement of pathogenic species, like Enterobacter spp [207-209]. Dietary carbohydrates regulate nervous system function. Vagus nerve, acting as the communication pathway between gut, live and brain senses peripheral glucose availability to the brain sites. Intake of carbohydrates also enhances tryptophan concentration in the brain [202]. Enteroendocrine cells can sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways [210]. Furthermore, glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake by CNS-GIPR signaling [211]. In situations of nerve injuries, nutrients show neuroprotective properties, apart from recovery of injured nerve tissue [212].
Caffeine helps to improve memory power by increasing cAMP (cyclic Adenosine monophosphate) levels. Curcumin from turmeric crosses the blood-brain barrier, boosts serotonin, dopamine and neutrophic factor and activates NRF2. This helps in new brain cell growth and is beneficial in animal models of traumatic brain injury, stroke, AD and PD. However, turmeric constitutes only 3-6% of curcumin [213-216]. The peripheral nervous system, susceptible to injury, can be regenerated by following a tailored diet [217].
Magnesium, involved in different and many enzymatic reactions leads to regeneration of the peripheral nerves [218]. Plant derived compounds can help in regeneration of the peripheral nerves [219]. Axonal regrowth in the nerve cells can be affected by DHA, part of neuronal membrane phospholipid by elevating expression of Bcl-2, as well as by inhibiting caspase 3-downstream effects [220-222]. Vitamin D expresses regulatory effect on neutrophic factors involved in nerve regeneration [223-225]. Polyphenols and polyphenol-rich foods benefit the regeneration of peripheral nerves and give neuroprotective effects in neuro degenerative diseases [226,227]. Methylcobalamin (MeCbl), activated form of vitamin B12, improves nerve conduction, regenerates nerves from injuries and is used in treatment of Ad and rheumatoid arthritis [228]. Also, a high fat diet can lead to hypothalamic dysfunction [229]. There are many nutrients that are beneficial for neuroprotective effect and brain functioning [230]. Ageing populations need brain health supplements. Vitamins C, D and B12 offer neuroprotective support and B family vitamins prevent dementia and boosts neurotransmitter production [229]. A few of those that affect the neural health of a person have been listed in Table 1 [231-239].
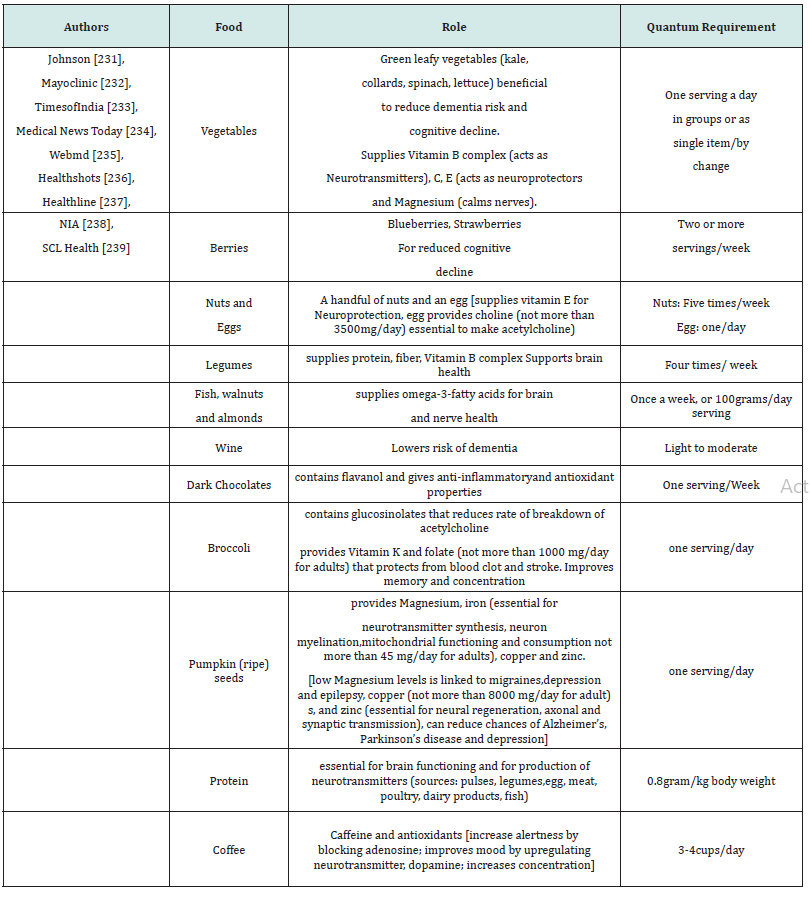
Table 1:Essential food ingredients for healthy neuro functioning. The Table highlights a few of the essential ingredients required in diet for neuroprotective functioning.
The probable dosage as per the recommendations and traditional culinary knowledge has been put forth over in Table 1, but one needs to look for quantum availability of that dosage from food product(s) as per the first line of therapy. Though there is less scientific evidence, Bacopa monnieri is consumed to improve brain health [240]. In general, the beneficial nutrients for balanced neurotransmitter supply to the brain includes amino acids, glucose, vitamins C, E, D, B-complex, β-carotene, minerals, arachidonic acids, docosahexaenoic acid, eicosapentaenoic acid, gamma-linolenic acid, apart from antioxidants for neuroprotective functions [241].
The presence of antioxidants in the diet protects against oxidative damage to nervous system cells. Biochemical data indicate that polyunsaturated fatty acids such as arachidonic acid (AA), docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and gamma-linolenic acid (GLA) as structural components of the nervous system play a key role in its function. The nutrition of the entire body also influences the production of neurotransmitters in the brain.
Conclusion
Therapeutic approaches have been adopted in patients with nervous system impairment using electrical stimulation of the CNS. However, it is unclear about the cells or cellular elements that activated upon electrical stimulation of the CNS [242]. There are many reports detailing the signaling and transcription factors that play a critical role in neural progenitor differentiation to neurons and glia cells. Even, long term memory is fuelled by pentose phosphate pathway of glial glucose [243]. Therapeutic strategies for ALS can stem out from glial mitochondrial function and metabolic reprogramming [244]. Development and induction of stem cells can help in replacement of the specific cells affected by neurodegenerative disease and injuries [245]. Direct regeneration of the neurons is a difficult process, however research understandings from the effect of growth factors, or any other component from the diet, or food component including lifestyle may help in regeneration studies under in vivo and in vitro conditions. The knowledge can be useful in the treatment of brain tumours also. The most common brain tumours in adults are glioma, and neuroblastoma in children. Loss of miRNA-34a leads to the loss of p53 that directs formation of adrenergic fibers in head and neck cancer [246]. Reports have identified molecular targets for therapeutical recovery procedures in brain tumours [247]. Experimenters have also uncovered a new class of memory cells, ‘grandmother neuron’ that is the crossroads of sensory perception and memory [248]. Exogenously administered growth factors also led to regeneration of peripheral nerves [249]. Reports also confirm the reversal of neurodegeneration at advanced stage by neuronal metabolic rewiring [250]. Furthermore, calmodulin kinases may also contribute to the pathology of neuropsychological disorders. Stress coupled with neuroinflammation leads to neurodegeneration. Herbal medicine in nerve regeneration is a therapeutic option in this situation, and Ginkgo biloba, an antioxidant can help in nerve regeneration. Higher dosage of Ginkgo biloba extract reported better results [251-254]. Lastly, neurons adjust fuel utilization under conditions of pathogenic reprogramming in amyotrophic lateral sclerosis (ALS) [255]. Metabolic reprogramming in astrocytes can distinguish region specific neuronal susceptibility [256].
The physical way of brain communication occurs via neurons. Mapping the total circuitry can help one to understand different functions, like cognitive functions. The question arises here, if food components, or any activities can lead to rewiring of the circuitry in adult brain? Can these components lead to neurogenesis? It is certain that regulatory pathways influence the process of neurogenesis. There is also dependence on mitochondrial activity, energy consumption and Gibb’s energy consumption [257,258]. Mitochondria also serves as the central regulator in the process of neurodifferentiation and for neural stem cell fate decisions [259]. Glial metabolites can lead to glial reprogramming to facilitate morphological and functional regeneration in the central nervous system after injury [260]. Neuroplasticity or brain plasticity shapes brain morphology and physiology, and neuroplasticity is regulated by intrinsic and extrinsic stimuli [261]. The hypothesis that can be put forth here is that can dietary components or physical and or social activities have a direct influence on the process of neurogenesis and neuroplasticity? Nevertheless, it is essential to understand the molecular effects of herbs, or any other food components to support the first line of therapy in treatments for nerve injury. The knowledge gained will also help to understand metabolic reprogramming in nerve cells. With the understanding of metabolic reprogramming mechanism in nerve cells, therapeutic possibilities using food, social interactions, exercise with the first line of therapy can highlight upon the curative options for neurodegenerative diseases. The axonal regeneration is regulated by intrinsic and extrinsic factors; however, the question remains whether any specific dietary components initiate or stimulate axonal regeneration. The hypothesis can also be experimented in cultured stem cell conditions to allow manipulation of NSCs for therapeutic uses. It is thus essential to understand molecular basis of the effect of diet on food cognition to manipulate diet for sustaining neuron health.
References
- (2023) Introduction to the nervous system. NCI Seer Training modules.
- (2023) Nerve Cells. Mayoclinic.
- (2020). Nervous System. Cleveland Clinic.
- Northcutt RG, Noback CR, Kallen B (2020) Nervous system (vertebrate). In: Access Science. McGraw Hill.
- Beckers L, Stroobants S, D Hooge R, Baes M (2018) Neuronal Dysfunction and Behavioral Abnormalities Are Evoked by Neural Cells and Aggravated by Inflammatory Microglia in Peroxisomal β-Oxidation Deficiency. Front Cell Neurosci 12:136.
- Lee Y, Lee H S, Kim M, Shin H (2020) Brain Cytoplasmic RNAs in Neurons: From Biosynthesis to Function. Biomolecules 10(2): 313.
- Faubert B, Solmonson A, Deberardinis RJ (2020) Metabolic reprogramming and cancer progression. Science. 368(6487): eaaw5473.
- Shuvalov O, Daks A, Fedorova O, Petukhov A, Barlev N (2021) Linking Metabolic Reprogramming, Plasticity and Tumor Progression. Cancers 13(4): 762.
- Ratnikov BI, Scott DA, Osterman AL, Smith JW, Ronai ZA (2017) Metabolic rewiring in melanoma. Oncogene 36(2): 147-157.
- (2023) Metabolic plasticity in health and diseases. Gustave Roussy Cancer Campus.
- Al Masri M, Paliotti K, Tran R, Ruba Halaoui, Virginie Lelarge, et al. (2021) Architectural control of metabolic plasticity in epithelial cancer cells. Communications Biology 4: 371.
- Cazzaniga M, Bonanni B (2015) Relationship between metabolic reprogramming and mitochondrial activity in cancer cells. Understanding the anticancer effect of metformin and its clinical implications. Anticancer Research 35 (11): 5789-5796.
- Paudel BB, Quaranta V (2019) Metabolic plasticity meets gene regulation. Proc Natl Acad Sci 116(9): 3370-3372.
- Yoshida GJ (2015) Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res 34: 111.
- (2015) Mapping energy metabolism of growing nerve cells to better understand neuronal disorders. ScienceDaily.
- Turner DA, Adamson DC (2011) Neuronal-Astrocyte Metabolic Interactions: Understanding the Transition into Abnormal Astrocytoma Metabolism. J Neuropathol Exp Neurol 70(3): 167-176.
- Kawabe H, Neeb A, Dimova K, Samuel M Young, Michiko Takeda, et al. (2010) Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development in cortical neurons. Neuron 65(3): 358-372.
- Weishaupt N, Zhang A (2016) Why doesn’t your brain heal like your skin? Front. Young Minds 4: 22.
- Önger ME, Delibaş B, Türkmen AP, Erener E, Altunkaynak BZ, et al. (2017) The role of growth factors in nerve regeneration. Drug Discoveries & Therapeutics 10(6): 285-291.
- Persengiev SP, Kondova I I, Kilpatrick DL (1999) E2F4 actively promotes the initiation and maintenance of nerve growth factor-induced cell differentiation. Mol Cell Biol 19(9): 6048-6056.
- Agrawal A, Pekkurnaz G, Koslover EF (2018) Spatial control of neuronal metabolism through glucose-mediated mitochondrial transport regulation. eLife 7: e40986.
- (2023) Neuronal Firing. Centre for NeuroSkills.
- Deitmer JW, Theparambil SM, Ruminot I, Noor SI, Becker HM (2019) Energy Dynamics in the Brain: Contributions of Astrocytes to Metabolism and pH Homeostasis. Front Neurosci 13:1301.
- Bonvento G, Bolanos JP (2021) Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab 33(8): 1546-1564.
- Mason S (2017) Lactate shuttles in neuroenergetics-homeostasis allostasis and beyond. Front Neurosci 11:43.
- Falkowska A, Gutowska I, Goschorska M, Nowacki P, Chlubek D, et al. (2015) Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int J Mol Sci 16(11): 25959-25981.
- Sagar SM, Sharp FR, Swanson RA (1987) The regional distribution of glycogen in rat brain fixed by microwave irradiation. Brain Res 417: 172-174.
- Vannucci SJ, Maher F, Simpson IA (1997) Glucose transporter proteins in brain: Delivery of glucose to neurons and glia. Glia 21(1): 2-21.
- Nehlig A (2004) Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot Essent Fat Acids 70(3): 265-275.
- Hertz L, Peng L, Dienel GA (2007) Energy metabolism in astrocytes: High rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab 27(2): 219-249.
- DiNuzzo M, Mangia S, Maraviglia B, Giove F (2013) Regulatory mechanisms for glycogenolysis and K+ uptake in brain astrocytes. Neurochem Int 63(5): 458-464.
- Choi HB, Gordon GR, Zhou N, Chao Tai, Ravi L Rungta, et al. (2012) Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron 75(6): 1094-1104.
- Xu J, Song D, Xue Z, Gu L, Hertz L, et al. (2013) Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: Potential implications for K+ homeostasis and glycogen usage in brain. Neurochem Res 38(3): 472-485.
- Yellen G (2018) Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J Cell Biol 217(7): 2235-2246.
- Turner DA, Adamson DC (2011) Neuronal-astrocyte metabolic interactions: understanding the transition into abnormal astrocytoma metabolism. J Neuropathol Exp Neurol 70 (3):167-176.
- Jha MK, Morrison BM (2018) Glia-neuron energy metabolism in health and diseases: New insights into the role of nervous system metabolic transporters. Exp Neurol 309: 23-31.
- Froes MM, Correia AH, Garcia-Abreu J, Spray DC, Carvalho AC de C, et al. (1999) Gap-junctional coupling between neurons and astrocytes in primary central nervous system cultures. Proc Natl Acad Sci USA 96(13): 7541-7546.
- Parpura V, Heneka MT, Montana V, Stéphane H R Oliet, Arne Schousboe, et al. (2012) Glial cells in (patho)physiology. J Neurochem 121 (1): 4-27.
- Vega C, Poitry Yamate CL, Jirounek P, Tsacopoulos M, Coles JA (1998) Lactate is released and taken up by isolated rabbit vagus nerve during aerobic metabolism. J Neurochem 71(1): 330-337.
- Brown AM, Evans RD, Black J, Ransom BR (2012) Schwann cell glycogen selectively supports myelinated axon function. Ann Neurol 72 (3): 406-418.
- Evans RD, Brown AM, Ransom BR (2013) Glycogen function in adult central and peripheral nerves. J Neurosci Res 91(8): 1044-1049.
- Hu X, Hou H, Bastian C, Wanxia He, Shupeng Qiu, et al. (2017) BACE1 regulates the proliferation and cellular functions of Schwann cells. Glia 65(5): 712-726.
- Stecker MM, Stevenson MR (2015) Anoxia-induced changes in optimal substrate for peripheral nerve. Neuroscience 284: 653-667.
- Diamond I, Nagy L, Mochly Rosen D, Gordon A (1991) The role of adenosine and adenosine transport in ethanol-induced cellular tolerance and dependence. Possible biologic and genetic markers of alcoholism. Ann N Y Acad Sci 625: 473-487.
- Kiselevski Y, Oganesian N, Zimatkin S, Szutowicz A, Angielski S, et al. (2003) Acetate metabolism in brain mechanisms of adaptation to ethanol. Med Sci Monit 9(5): BR178-182.
- Kiviluoma KT, Peuhkurinen KJ, Hassinen IE (1989) Adenine nucleotide transport and adenosine production in isolated rat heart mitochondria during acetate metabolism. Biochim Biophys Acta 974 (3): 274-281.
- Mailliard WS, Diamond I (2004) Recent advances in the neurobiology of alcoholism: the role of adenosine. Pharmacol Ther 101 (1): 39-46.
- Williams NC, O’Neill LAJ (2018) A role for the krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front Immunol 9: 141.
- Diaz Garcia CM, Mongeon R, Lahmann C, Koveal D, Zucker H, et al. (2017) Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab 26 (2): 361-374.e4.
- Magistretti PJ, Allaman I (2018) Lactate in the brain: from metabolic end-product to signaling molecule. Nat Rev Neurosci 19(4): 235-249.
- Teng J, Rai T, Tanaka Y, Yosuke Takei, Takao Nakata, et al. (2005) The KIF3 motor transports N-cadherin and organizes the developing neuroepithelium. Nat Cell Biol 7(5): 474-482.
- Hirokawa N, Niwa S, Tanaka Y (2010) Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68(4): 610-638.
- Amjad S, Nisar S, Bhat AA, Ab Rauf Shah, Michael P. Frenneaux, et al. (2021) Role of NAD+ in regulating cellular and metabolic signaling pathways. Molecular Metabolism 49: 101195.
- Plaitakis A, Kalef Ezra E, Kotzamani D, Zaganas l, Spanaki C, et al. (2017) The glutamate dehydrogenase pathway and its roles in cell and tissue biology in health and disease. Biology (Basel) 6(1):11.
- Tsacopoulos M, Poitry Yamate CL, Poitry S (1997). Ammonium and glutamate released by neurons are signals regulating the nutritive function of a glial cell. J Neurosci 17(7): 2383-2390.
- Coyle JT, Balu D, Wolosker H (2020) D-Serine the shape-shifting NMDA receptor co-agonist. Neurochem Res 45(6): 1344-1353.
- Martineau M, Parpura V, Mothet JP (2014) Cell-type specific mechanisms of D-serine uptake and release in the brain. Front Synaptic Neurosci 6: 12.
- Mothet JP, Parent AT, Wolosker H, RO Brady, DJ Linden, et al. (2000) D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA 97(9): 4926-4931.
- Papouin T, Ladepeche L, Ruel J, Silvia Sacchi, Marilyne Labasque, et al. (2012) Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150(3): 633-646.
- Rajendra S, Lynch JW, Schofield PR (1997) The glycine receptor. Pharmacol Ther 73(2): 121-146.
- Ducker GS, Rabinowitz JD (2017) One-carbon metabolism in health and disease. Cell Metab 25(1): 27-42.
- Hertz L, Peng L, Dienel GA (2007) Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab 27(2):219-249.
- Brown AM, Sickmann HM, Fosgerau K, Trine M Lund, Arne Schousboe, et al. (2005) Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J Neurosci Res 79(1-2): 74-80.
- Gruetter R (2003) Glycogen: the forgotten cerebral energy store. J Neurosci Res 74(2): 179-83.
- Kim H-Y, Huang BX, Spector AA (2014) Phosphatidylserine in the Brain: Metabolism and Function. Prog Lipid Res 56: 1-18.
- Andersen JV, Markussen KH, Jakobsen E, Arne Schousboe, Helle S Waagepetersen, et al. (2021) Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology. Neuropharmacology 196: 108719.
- Dienel GA (2019) Brain glucose metabolism: integration of energetics with function. Physiol Rev 99(1): 949-1045.
- McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U (2006) Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools. Biochem Pharmacol 71(4): 399-407.
- Robinson SR (2000) Neuronal expression of glutamine synthetase in Alzheimer’s disease indicates a profound impairment of metabolic interactions with astrocytes. Neurochem Int 36(4-5): 471-482.
- Smith CD, Carney JM, Starke Reed PE, C N Oliver, E R Stadtman, et al. (1991) Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA 88(23): 10540-10543.
- Camandola S, Mattson MP (2017) Brain metabolism in health, aging and neurodegeneration. The EMBO Journal 36 (11): 1474-1492.
- Kubotera H, Ikeshima Kataoka H, Hatashita Y, Mascaro ALA, Pavone FS, et al. (2019) Astrocytic endfeet re-cover blood vessels after removal by laser ablation. Sci Rep 9(1): 1263.
- Belanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab 14(7): 724-738.
- Lebon V, Petersen KF, Cline GW, Jun Shen, Graeme F Mason, et al. (2002) Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci 22(5): 1523-1531.
- Boumezbeur F, Mason GF, de Graaf RA, Kevin L Behar, Gary W Cline, et al. (2010) Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab 30(1): 211-221.
- Bouzier Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, et al. (2006) Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci 24(6): 1687-1694.
- Itoh Y, Esaki T, Shimoji K, Michelle Cook, Mona J Law, et al. (2003) Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci USA 100(8): 4879-4884.
- Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62(6): 649-671.
- Watts ME, Pocock R, Claudianos C (2018) Brain energy and oxygen metabolism: emerging role in normal function and disease. Front Mol Neurosci 11: 216.
- Almeida A, Almeida J, Bolanos JP, Moncada S (2001) Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci USA 98(26): 15294-15299.
- Herrero Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, et al. (2009) The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol 11(6): 747-752.
- Vilchez D, Ros S, Cifuentes D, Lluís Pujadas, Jordi Vallès, et al. (2007) Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci 10(11): 1407-1413.
- Magistretti PJ, Allaman I (2015) A cellular perspective on brain energy metabolism and functional imaging. Neuron 86(4): 883-901.
- Allaman I, Belanger M, Magistretti PJ (2011) Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci 34(2): 76-87.
- Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA (2002) Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem 80(1): 91-100.
- Wirths O, Multhaup G, Czech C, V Blanchard, S Moussaoui, et al. (2001) Intraneuronal Aβ accumulation precedes plaque formation in β -amyloid precursor protein and presenilin-1 double transgenic mice. Neurosci Lett 306(1-2): 116-120.
- Du H, Guo L, Yan S, Sosunov AA, McKhann GM, et al. (2010) Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Natl Acad Sci USA 107(43): 18670-18675.
- Behl C, Davis JB, Lesley R, Schubert D (1994) Hydrogen peroxide mediates amyloid β protein toxicity. Cell 77(6): 817-827.
- Harris ME, Hensley K, Butterfield DA, Leedle RA, Carney JM (1995) Direct evidence of oxidative injury produced by the Alzheimer’s β-amyloid peptide (1-40) in cultured hippocampal neurons. Exp Neurol 131(2): 193-202.
- Bianca VD, Dusi S, Bianchini E, Dal Prà I, Rossi F (1999) β-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. J Biol Chem 274: 15493-15499.
- Sun X, He G, Qing H, Weihui Zhou, Frederick Dobie, et al. (2006) Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci USA 103(49): 18727-18732.
- Suh SW, Jensen KB, Jensen MS, DS Silva, PJ Kesslak, et al. (2000) Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res 852(2): 274-278.
- Schrag M, Mueller C, Oyoyo U, Smith MA, Kirsch WM (2011) Iron, zinc and copper in the Alzheimer’s disease brain: a quantitative metaanalysis. Some insight on the influence of citation bias on scientific opinion. Prog Neurobiol 94(3): 296-306.
- Ventriglia M, Bucossi S, Panetta V, Squitti R (2012) Copper in Alzheimer’s disease: a meta-analysis of serum, plasma, and cerebrospinal fluid studies. J Alzheimers Dis 30(4): 981-984.
- Miller LM, Wang Q, Telivala TP, Smith RJ, Lanzirotti A, et al. (2006) Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with b-amyloid deposits in Alzheimer’s disease. J Struct Biol 155(1): 30-37.
- Smith MA, Harris PL, Sayre LM, Perry G (1997) Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA 94(18): 9866-9868.
- Raven EP, Lu PH, Tishler TA, Heydari P, Bartzokis G (2013) Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer’s disease detected in vivo with magnetic resonance imaging. J Alzheimers Dis 37(1): 127-136.
- Bartzokis G, Sultzer D, Cummings J, L E Holt, D B Hance, et al. (2000) in vivo evaluation of brain iron in Alzheimer disease using magnetic resonance imaging. Arch Gen Psychiatry 57(1): 47-53.
- Ayton S, Faux NG, Bush AI, and Alzheimer’s Disease Neuroimaging Initiative (2015) Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun 6: 6760.
- Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219(4587): 979-980.
- Parker WD Jr, Parks JK (2005) Mitochondrial ND5 mutations in idiopathic Parkinson’s disease. Biochem Biophys Res Commun 326(3): 667-669.
- Plun Favreau H, Klupsch K, Moisoi N, Sonia Gandhi, Svend Kjaer, et al. (2007) The mitochondrial protease HtrA2 is regulated by Parkinson’s disease associated kinase PINK1. Nat Cell Biol 9(11): 1243-1252.
- van de Hall G, Stromstad M, Rasmussen P, Ole Jans, Morten Zaar, et al. (2009) Blood lactate is an important energy source for the human brain. J Cereb Flow Metab 29(6): 1121-1129.
- Nehlig A (2004) Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot Essent Fatty Acids 70(3): 265-275.
- Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR (1997) Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol 273(1 pt 1): E207-E213.
- Pellerin L, Pellegri G, Martin JL, Magistretti PJ (1998) Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci USA 95(7): 3990-3995.
- Yeh YY, Streuli VL, Zee P (1977) Ketone bodies serve as important precursors of brain lipids in the developing rat. Lipids 12(11): 957-964.
- Linares A, Caamano GJ, Diaz R, Gonzalez FJ, Garcia Peregin E (1993) Utilization of ketone bodies by chick brain and spinal cord during embryonic and postnatal development. Neurochemical Res 18(10): 1107-1112.
- Edmond J (1992) Energy metabolism in developing brain cells. Can J Physiol Pharmacol 70(Suppl): S118-S129.
- Sokoloff L (1973) Changes in enzyme activities in neural tissues with maturation and development of the nervous system. In: Schmidt FO (ed) The neurosciences third study program, Cambridge, USA, MIT Press, pp 885-898.
- LaManna JC, Salem N, Puchowicz M, Bernadette Erokwu, Smruta Koppaka, et al. (2009) Ketones suppress brain glucose consumption. Adv Exp Med Biol 645: 301-306.
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, et al. (1967) Brain metabolism during fasting. J Clin Invest 46(10): 1589-1595.
- Takaku Y, Hwang JS, Wolf A, Angelika Böttger, Hiroshi Shimizu, et al (2014) Innexin gap junctions in nerve cells coordinate spontaneous contractile behavior in Hydra polyps. Scientific Reports 4: 3573.
- Nakamura S, Akiguchi I, Kameyama M, Mizuno N (1985) Age-related changes of pyramidal cell basal dendrites in layers III and V of human motor cortex: a quantitative Golgi study. ActaNeuropathol 65(3-4): 281-284.
- Page TL, Einstein M, Duan H, Yong He, Tony Flores, et al. (2002) Morphological alterations in neurons forming corticocortical projections in the neocortex of aged Patas monkeys. Neurosci Lett 317(1): 37-41.
- Guttmann CR, Jolesz FA, Kikinis R, R J Killiany, M B Moss, et al. (1998) White matter changes with normal aging. Neurology 50(4): 972-978.
- Stahon KE, Bastian C, Griffith S, Kidd GJ, Brunet S, et al. (2016) Age-related changes in axonal and mitochondrial ultrastructure and function in white matter. J Neurosci 36(39): 9990-10001.
- Hoyer S (1982) The young adult and normally aged brain. Its flow and oxidative metabolism. A review-part I. Arch Gerontol Geriatr 1(2): 101-116.
- Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W (2015) In vivo NAD assay reveals the intracellular NAD content and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci USA 112(9): 287628-287681.
- Harris JJ, Jolivet R, Attwell D (2012) Synaptic energy use and supply. Neuron 75(5): 762-777.
- Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12: 723-738.
- Winkler EA, Nishida Y, Sagare AP, Sanket V Rege, Robert D Bell, et al. (2015) GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 18(4): 521-530.
- Niebroj-Dobosz I, Janik P, Sokołowska B, Kwiecinski H (2010) Matrix metalloproteinases and their tissue inhibitors in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Eur J Neurol 17(2): 226-231.
- Miyazaki K, Ohta Y, Nagai M, Nobutoshi Morimoto, Tomoko Kurata, et al. (2011) Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J Neurosci Res 89(5): 718-728.
- Leonardi A, Abbruzzese G, Arata L, Cocito L, Vische M (1984) Cerebrospinal fluid (CSF) findings in amyotrophic lateral sclerosis. J Neurol 231(2): 75-78.
- Annunziata P, Volpi N (1985) High levels of C3c in the cerebrospinal fluid from amyotrophic lateral sclerosis patients. Acta Neurol Scand 72(1): 61-64.
- Donnenfeld H, Kascsak RJ, Bartfeld H (1984) Deposits of IgG and C3 in the spinal cord and motor cortex of ALS patients. J Neuroimmunol 6(1): 51-57.
- Pradat PF, Bruneteau G, Gordon PH, Luc Dupuis, Dominique Bonnefont-Rousselot, et al. (2010) Impaired glucose tolerance in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 11(1-2): 166-171.
- Reyes ET, Perurena OH, Festoff BW, Jorgensen R, Moore WV (1984) Insulin resistance in amyotrophic lateral sclerosis. J Neurol Sci 63(3): 317-324.
- Dupuis L, Corcia P, Fergani A, J L Gonzalez De Aguilar, D Bonnefont Rousselot, et al. (2008) Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70(13): 1004-1009.
- Pickrell AM, Youle RJ (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85(2): 257-273.
- Randerath W, Bassetti CL, Bonsignore MR, Luigi Ferini Strambi, Ludger Grote, et al. (2018) Challenges and perspectives in obstructive sleep apnoea. Eur Respir J 52(3): 1702616.
- Koritala BSC, Conroy Z, Smith DF (2021) Circadian biology in obstructive sleep apnea. Diagnostics (Basel) 11(6): 1082.
- White DP (2006) The pathogenesis of obstructive sleep apnea. Advances in the past 100 years. Am J Respir Cell Mol Biol 34(1): 1-6.
- Martínez Espinosa RM, Vila MDM, García Galbis MR (2020) Evidences from clinical trials in down syndrome: diet, exercise and body composition. Int J Environ Res Public Health 17(12): 4294.
- (2008) Molecular Biology of sleep apnea could lead to new treatments. ScienceDaily.
- Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI (2009) Molecular signatures of obstructive sleep apnea in adults: A review and perspective. SLEEP 32(4): 447-470.
- Gasco S, Murenu E, Masserdotti G, Felipe Ortega, Gianluca L Russo, et al. (2016) Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem Cell 18(3): 396-409.
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G (2014) In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 14(2): 188-202.
- Renthal W, Tocjitsky I, Yang L, Yung Chih Cheng, Emmy Li, et al. (2020) Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron 108(1): 128-144.e9.
- Lahne M, Nagashima M, Hyde DR, Hitchcock PF (2020) Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu Rev Vis Sci 6: 171-193.
- Cai XT, Li H, Jensen MB, Elie Maksoud, Jovencio Borneo, et al. (2021) Gut cytokines modulate olfaction through metabolic reprogramming of glia. Nature 596(7870): 97-102.
- Cassina P, Miquel E, Martinez Palmaa L, Cassina A (2021) Glial metabolic reprogramming in amyotrophic lateral sclerosis. Neuroimmunomodulation 28(4): 204-212.
- Sonnino S, Prinetti A (2016) The role of sphingolipids in neuronal plasticity of the brain. Journal of Neurochemistry 137(4): 485-488.
- Li X, Zhang J, Li D, Cheng He, Keqiang He, et al. (2021) Astrocytic ApoE reprograms neuronal cholesterol metabolism and histone-acetylation-mediated memory. Neuron 109(6): 957-970.
- Önger ME, Delibas B, Türkmen AP, Erener E, Altunkaynak BZ, et al. (2016) The role of growth factors in nerve regeneration. Drug Discoveries & Therapeutics 10(6): 285-291.
- Quiroz EN, Quiroz RN, Ahmad M, Lorena Gomez Escorcia, Jose Luis Villarreal, et al. (2018) Cell Signaling in Neuronal Stem Cells. Cells 7(7): 75.
- Rogel A, Popliker M, Webb CG, Oren M (1985) p53 cellular tumor antigen: Analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol 5: 2851-2855.
- Komarova EA, Chernov MV, Franks R, K Wang, G Armin, et al. (1997) Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J 16(6): 1391-1400.
- Campagne MVL, Gill R (1998) Tumor-suppressor p53 is expressed in proliferating and newly formed neurons of the embryonic and postnatal rat brain: Comparison with expression of the cell cycle regulators p21Waf1/Cip1, p27Kip1, p57Kip2, p16Ink4a, cyclin G1, and the proto-oncogene Bax. J Comp Neurol 397(2): 181-198.
- Yang A, Walker N, Bronson R M Kaghad, M Oosterwegel, et al. (2000) p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404(6773): 99-103.
- Fuertes Alvarez S, Maeso Alonso L, Villoch Fernandez J, Merit Wildung, Marta Martin Lopez, et al. (2018) p73 regulates ependymal planar cell polarity by modulating actin and microtubule cytoskeleton. Cell Death Dis 9: 1183.
- Ziemka Nalecz M, Jaworska J, Sypecka J, Zalewska T (2018) Histone deacetylase inhibitors: a therapeutic key in neurological disorders? J Neuropathol Exp Neurol 77(10): 855-870.
- Tee AR, Sampson JR, Pal DK, Bateman JM (2016) The role of mTOR signalling in neurogenesis, insights from tuberous sclerosis complex. Semin Cell Dev Biol 52: 12-20.
- Bove J, Martinez Vicente M, Vila M (2011) Fighting neurodegeneration with rapamycin: Mechanistic insights. Nat Rev Neurosci 12(8): 437-452.
- Niklison Chirou MV, Agostini M, Amelio I, Melino G, Gerry Melino (2020) Regulation of adult neurogenesis in mammalian brain. Int J Mol Sci 21(14): 4869.
- Zoidl GR, Spray DC (2021) The roles of calmodulin and CaMKII in Cx36 plasticity. Int J Mol Sci 22(9): 4473.
- Sawada J, Itakura A, Tanaka A, Furusaka T, Matsuda H (2000) Nerve growth factor functions as a chemoattractant for mast cells through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Blood 95(6): 2052-2058.
- Gage FH (2000) Mammalian neural stem cells. Science 287(5457): 1433-1438.
- Shi Y, Sun G, Zhao C, Stewart R (2008) Neural stem cell self-renewal. Crit Rev Oncol Hematol 65(1): 43-53.
- Chae, TH, Kim S,Marz KE, Hanson PI, Walsh CA (2004) The hyh mutation uncovers roles for alpha Snap in apical protein localization and control of neural cell fate. Nat Genet 36(3): 264-270.
- Mangelsdorf DJ, Thummel C, Beato M, P Herrlich, G Schütz, et al. (1995) The nuclear receptor superfamily: the second decade. Cell 83(6): 835-839.
- Nishino J, Kim I, Chada K, Morrison SJ (2008) Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf expression. Cell 135(2): 227-239.
- Snider WD (1994) Functions of neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77(5): 627-638.
- Lewin GR, Barde Y A (1996) Physiology of the neurotrophins. Annu Rev Neurosci 19: 289-317.
- Glebova NO, Ginty DD (2005) Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci 28: 191-222.
- Zweifel LS, Kuruvilla R, Ginty DD (2005) Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci 6(8): 615-625.
- Lee H K, Lee H S, Moody SA (2014) Neural transcription factors: from embryos to neural stem cells. Mol Cells 37(10): 705-712.
- Tutukova S, Tarabykin V, Hernandez Miranda LR (2021) The role of neurod genes in brain development, function, and disease. Front Mol Neurosci 14: 662774.
- Pataskar A, Jung J, Smialowski P, Florian Noack, Federico Calegari, et al. (2016) NeuroD1 reprograms chromatin and transcription factor landscapes to induce the neuronal program. EMBO J 35(1): 24-45.
- Zhang Q, Huang R, Ye Y, Xiaoxia Guo, Jun Lu, et al. (2018) Temporal requirements for ISL1 in sympathetic neuron proliferation, differentiation, and diversification. Cell Death and Disease 9(2): 247.
- Letourneau PC, Shattuck TA (1989) Distribution and possible interactions of actin-associated proteins and cell adhesion molecules of nerve growth cones. Development 105(3): 505-519.
- Saito A, Cavalli V (2016) Signaling over distances. Mol Cell Proteomics 15(2): 382-393.
- Soret R, Schneider S, Bernas G, Briana Christophers, Ouliana Souchkova, et al. (2020) Glial cell-derived neurotrophic factor induces enteric neurogenesis and improves colon structure and function in mouse models of Hirschsprung disease. Gastroenterology 159(5): 1824-1838.e17.
- Yu T, Xu Y, Ahmad MA, Javed R, Hagiwara H, et al. (2021) Exosomes as a promising therapeutic strategy for peripheral nerve injury. Curr Neuropharmacol 19(12): 2141-2151.
- Sanz Ezquerro JJ, Münsterberg AE, Stricker S (2017) Editorial: Signaling Pathways in Embryonic Development. Front Cell Dev Biol 5:76.
- Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, et al. (2004) IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol 164(1): 111-122.
- Yasuhara T, Shingo T, Date I (2004) The potential role of vascular endothelial growth factor in the central nervous system. Rev Neurosci 15(4): 293-307.
- Cao L, Jiao X, Zuzga DS, Yuhong Liu, Dahna M Fong, et al. (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36(8): 827-835.
- Michalski JP, Kothary R (2015) Oligodendrocytes in a nutshell. Front Cell Neurosci 9: 340.
- Behar TN, Schaffner AE, Scott CA, O’Connell C, Barker JL (1998) Differential response of cortical plate and ventricular zone cells to GABA as a migration stimulus. J Neurosci 1998; 18(16): 6378-6387.
- Sommer B, Seeburg PH (1992) Glutamate receptor channels: novel properties and new clones. Trends Pharmacol Sci 13(7): 291-296.
- Owens DF, Kriegstein AR (2002) Is there more to gaba than synaptic inhibition? Nat Rev Neurosci 3(9): 715-727.
- Pendleton RG, Rasheed A, Roychowdhury R, Hillman R (1998) A new role for catecholamines: ontogenesis. Trends Pharmacol Sci 19(7): 248-251.
- Droz B, Barakat I, Kazimierczak J, Philippe E, Rochat A (1990) Remodeling of neuronal subpopulations in dorsal root ganglion: role of chemical factors and intercellular contacts.The primary afferent neuron 119-120.
- Koenig HL, Droz B (1971) Effect of nerve section on protein metabolism of ganglion cells and preganglionic nerve endings. Acta Neuropathol vol 5: 119-125.
- Uyeda A, Muramatsu R (2020) Molecular mechanisms of central nervous system axonal regeneration and remyelination: A review. Int J Mol Sci 21(21): 8116.
- He Z, Jin Y (2016) Intrinsic control of axon regeneration. Neuron 90(3): 437-451.
- Mattson MP (2015) Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev 20: 37-45.
- Hirano Y, Masuda T, Naganos S, Motomi Matsuno, Kohei Ueno, et al. (2013) Fasting launches CRTC to facilitate long-term memory formation in Drosophila. Science 339(6118): 443-446.
- Placais PY, Preat T (2013) To favor survival under food shortage, the brain disables costly memories. Science 339(6118): 440-442.
- Marini AM, Paul SM (1992) N-methyl-D-aspartate receptor-mediated neuroprotection in cerebellar granule cells requires new RNA and protein synthesis. Proc Natl Acad Sci USA 89(14): 6555-6559.
- Lee J, Bruce Keller AJ, Kruman Y, Chan SL, Mattson MP (1999) 2-Deoxy-Dglucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J Neurosci Res 57(1): 48-61.
- Liu D, Zhang Y, Gharavi R, Hee Ra Park, Jaewon Lee, et al. (2015) The mitochondrial uncoupler DNP triggers brain cell mTOR signaling network reprogramming and CREB pathway-up-regulation. J Neurochem 134(4): 677-692.
- Mattson MP (2012) Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab 16(6): 706-722.
- Marosi K, Bori Z, Hart N, L Sárga, E Koltai, et al. (2012) Long term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience 226: 21-28.
- Madinier A, Bertrand N, Rodier M A Quirié, C Mossiat, et al. (2013) Ipsilateral versus contralateral spontaneous post- stroke neuroplastic changes: involvement of BDNF? Neuroscience 231: 169-181.
- Bhattacharya S, Latha JNL, Kumresan R, Singh S (2007) Cloning and expression of human islet amyloid polypeptide in cultured cells. Biochem Biophys Res Commun 356(3): 622-628.
- Jadon N, Sabharwal N, Bhattacharya S (2019) Impact of cell senescence on age-related neurodegenerative disorders: the mechanism to therapy- a review. International Journal of Pharmaceutical Sciences and Research 10(5): 2101-2107.
- Lutz TA, Meyer U (2015) Amylin at the interface between metabolic and neurodegenerative disorders. Front Neurosci 9: 216.
- Kiriyama Y, Nochi H (2018) Role and cytotoxicity of amylin and protection of pancreatic islet β-cells from amylin cytotoxicity. Cells 7(8): 95.
- Gomez Pinilla F, Gomez AG (2011) The influence of dietary factors in central nervous system plasticity and injury recovery. PM R 3 (6 Suppl 1): S111-S116.
- Edmund TSL, Anderson GH (1987) Dietary carbohydrate and the nervous system. Nutrition Res 7(12): 1329-1339.
- Langley MR, Tripletb EM, Scarisbrick IA (2020) Dietary influence on central nervous system myelin production, injury, and regeneration. Biochim Biophys Acta - Mol Basis Disease 1866(7): 165779.
- Hedstrom AK, Bomfim IL, Hillert J, Olsson T, Alfredsson L (2015) Obesity interacts with infectious mononucleosis in risk of multiple sclerosis. Eur J Neurol 22 (3): 578-e38.
- Sicras Mainar A, Ruiz Beato E, Navarro Artieda R, Maurino J (2017) Comorbidity and metabolic syndrome in patients with multiple sclerosis from Asturias and Catalonia, Spain. BMC Neurol 17(1): 134.
- Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, et al. (2009) Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology 72(2): 117-124.
- Sushrut Jangi, Roopali Gandhi, Laura M Cox, Ning Li, Felipe von Glehn, et al. (2016) Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 7: 12015.
- Berer K, Gerdes LA, Cekanaviciute E, Xiaoming Jia, Liang Xiao, et al. (2017) Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci USA 114(40): 10719-10724.
- Tankou SK, Regev K, Healy BC, Emily Tjon, Luca Laghi, et al. (2018) A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol 83(6): 1147-1161.
- Ye L, Bae M, Chelsi D, Sairam V Jabba, Daniel W Thorpe, et al. (2021) Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 29(2): 179-196.e9.
- Zhang Q, Delessa CT, Augustin R, Mostafa Bakhti, Gustav Colldén, et al. (2021) The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab 33(4): 833-844.e5.
- Yildiran H, Macit SM, Uyar GO (2020) New approach to peripheral nerve injury: nutritional therapy. Nutr Neurosci 23(10): 744-755.
- Son TG, Camandola S, Mattson MP (2008) Hormetic dietary phytochemicals. Neuromolecular Med 10(4): 236-246.
- Son TG, Camandola S, Arumugam TV, Roy G Cutler, Richard S Telljohann, et al. (2010) Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem 112(5): 1316-1326.
- Mattson MP (2015) What doesn’t kill you. Sci Am 313: 40-45.
- Gómez Pinilla F (2008) Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 9(7): 568-578.
- Soury EM, Fornasari BE, Carta G, Federica Zen, Kirsten Haastert Talini, et al. (2021) The role of dietary nutrients in peripheral nerve regeneration. Int J Mol Sci 22(14): 7417.
- Zhang J, Zhang B, Zhang J, Lin W, Zhang S (2021) Magnesium promotes the regeneration of the peripheral nerve. Front Cell Dev Biol 9: 717854.
- Hussain G, Wang J, Rasul A, Haseeb Anwar, Muhammad Qasim, et al. (2020) Current status of therapeutic approaches against peripheral nerve injuries: A detailed story from injury to recovery. Int J Biol Sci 16(1): 116-134.
- Zendedel A, Habib P, Dang J, Cordian Beyer, Alexander Slowik, et al. (2015) Omega-3 polyunsaturated fatty acids ameliorate neuroinflammation and mitigate ischemic stroke damage through interactions with astrocytes and microglia. J Neuroimmunol 278: 200-211.
- Bazan NG (2009) Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J Lipid Res 50: S400-S405.
- Bazan NG (2009) Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot Essent Fat Acids 81(2-3): 205-211.
- Naveilhan P, Neveu I, Wion D, Brachet P (1996) 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport 7(13): 2171-2175.
- Neveu I, Naveilhan P, Jehan F, H F De Luca, P Brachet, et al. (1994) 1,25-Dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res Mol Brain Res 24(1-4): 70-76.
- Gomez Pinilla F (2008) Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 9(7): 568-578.
- Wion D, Macgrogan D, Neveu I, Jehan F, Houlgatte R, et al. (1991) 1,25- Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J Neurosci Res 28(1): 110-114.
- Muratori L, Fregnan F, Maurina M, Haastert Talini K, Ronchi G (2022) The potential benefits of dietary polyphenols for peripheral nerve regeneration. Int J Mol Sci 23(9): 5177.
- Zhang M, Han W, Hu S, Xu H (2013) Methylcobalamin: a potential vitamin of pain killer. Neural Plast 2013: 424651.
- Williams LM (2012) Hypothalamic dysfunction in obesity. Proc Nutr Soc 71(4): 521-533.
- Johnson R (2021) Five supplement ingredient trends set to dominate. Custom Capsule Consultants.
- (2021) Healthy lifestyle: Nutrition and healthy eating. Mayoclinic.
- (2018) Top 10 foods for brain and nervous system. Times of India.
- (2018) How much food should I eat each day? Medical News Today.
- (2022) Food sources for vitamins and minerals. WebMD.
- (2022) Vitamins and minerals for older adults. National Institute on Aging.
- (2022) 8 nutrients that can sharpen your brain and memory. Healthshots.
- (2021) 11 best foods to boost your brain and memory. Healthline.
- (2018) How much protein is simply too much? SCL Health.
- WebMD (2018) Bacopa-uses, side effects and more.
- Wendołowicz A, Stefańska E, Ostrowska L (2018) Influence of selected dietary components on the functioning of the human nervous system. Rocz Panstw Zakl Hig 69(1): 15-21.
- McIntyre CC, Grill WM (1999) Excitation of central nervous system neurons by nonuniform electric fields. Biophys J 76(2): 878-888.
- de Tredern E, Rabah Y, Pasquer L, Minatchy J, Plaçais PY, et al. (2021) Glial glucose fuels the neuronal pentose phosphate pathway for long-term memory. Cell Rep 36(8): 109620.
- Cassina P, Miquel E, Martinez Palma L, Cassina A (2021) Glial metabolic reprogramming in amyotrophic lateral sclerosis. Neuroimmunomodulation 28(4): 204-212.
- Lee HK, Lee HS, Moody SA (2014) Neural transcription factors: from embryos to neural stem cells. Mol Cells 37(10): 705-712.
- Amit M, Takahashi H, Dragomir MP, Carlos Caulin, Assaf Zinger, et al. (2020) Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 578: 449-454.
- Rich JN, Eyler CE (2008) Cancer stem cells in brain tumor biology. Cold Spring Harb Symp Quant Biol 73: 411.
- (2021) Scientists discover a new class of memory cells for remembering faces. ScienceDaily
- Li R, Li DH, Zhang HY, Wang J, Li XK, et al. (2020) Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol Sin 41(10): 1289-1300.
- Motori E, Atanassov I, Kochan SMV, J Puyal, NG Larsson, et al. (2020) Neuronal metabolic rewiring promotes resilience to neurodegeneration caused by mitochondrial dysfunction. Sci Adv 6(35): eaba8271.
- Takemoto Kimura S, Suzuki K, Horigane SI, Hajime Fujii, Haruhiko Bito, et al. (2017) Calmodulin kinases: essential regulators in health and disease. J Neurochem 141(6): 808-818.
- Ekici Gunay N (2020) Ginkgo biloba extract as an antioxidant in nerve regeneration. In: Oxidative stress and dietary antioxidants. Academic Press 235-245.
- Huang WL, Ma YX, Fan YB, Guo Ying Li, Su Min Tian, et al. (2017) Extract of Ginkgo biloba promotes neuronal regeneration in the hippocampus after exposure to acrylamide. Neural Regen Res 12(8): 1287-1293.
- Lin H, Wang H, Chen D, Gu Y (2007) A dose-effect relationship of Ginkgo biloba extract to nerve regeneration in a rat model. Microsurgery 27(8): 673-677.
- Riechers SP, Mojsilovic Petrovic J, Belton TB, Gerald Dienel, Robert G Kalb, et al. (2022) Neurons undergo pathogenic metabolic reprogramming in models of familial ALS. Mol Metab 60: 101468.
- Aris A Polyzos, Do Yup Lee, Rupsa Datta, Meghan Hauser, Helen Budworth, et al. (2019) Metabolic reprogramming in astrocytes distinguishes region-specific neuronal susceptibility in Huntington mice. Cell Metab 29(6): 1258-1273.
- Sophie H Bennett, Alastair J Kirby, Gerald T Finnerty (2018) Rewiring the connectome: evidence and effects. Neurosci Biobehav Rev 88: 51-62.
- Anna Maria Colangelo, Giovanni Cirillo, Lilia Alberghina, Michele Papa, Hans V Westerhoff (2019) Neural plasticity and adult neurogenesis: the deep biology perspective. Neural Regen Res 14(2): 201-205.
- Camilla Maffezzini, Javier Calvo Garrido, Anna Wredenberg, Christoph Freyer (2020) Metabolic regulation of neurodifferentiation in the adult brain. Cell Mol Life Sci 77(13): 2483-2496.
- Feng Li, Armin Sami, Harun N Noristani, Kieran Slattery, Jingyun Qiu, et al. (2020) Glial metabolic rewiring promotes axon regeneration and functional recovery in the central nervous system. Cell Metab 32(5): 767-785.
- Pedro Mateos Aparicio, Antonio Rodríguez Moreno (2019) The impact of studying brain plasticity. Front Cell Neurosci 13: 66.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.